Getting to the Heart of things
This week a lawsuit against the CDC has given us access to some of the V-safe data. This is the smartphone app created by the CDC that lets you register for the vaccine and report side effects and other health information. As with any user reported data it should be taken with a grain of salt but there are people who argue that reporting systems like this and VAERS undercount adverse events because most people don’t bother reporting. Either way this is data that the CDC has been sitting on and only released due to a recent court order. The data provided is from 10.1 million users and the headline number is 7.7% of those required medical attention after vaccination. It is such an absurdly high number that if I was working at the CDC, I would insist it was incorrect and there must be something wrong with the software. It will be very interesting moving forward to know how many employees were privy to this information or if there was any internal debate about releasing or debunking it. Data like this would create a liability nightmare, that is if liability existed for such a thing.
The dashboard for the data can be found here https://www.icandecide.org/v-safe-data/
The Florida surgeon general has also released a statement recently recommending that males 18-39 should not receive mRNA vaccines. Along with the statement was an analysis conducted by the state revealing an 84% increase of cardiac related incidence of death within 28 days of vaccination.
The guidance statement can be found here
The analysis itself is here
The most egregious example of a drug product causing excess heart attacks was not that long ago. Vioxx was brought to market in 1999 ironically as a safer alternative to traditional NSAID drugs like naproxen or aspirin which can cause ulcers and other gastrointestinal complications. In that respect the drug did work but the cardiovascular risks far outweighed that benefit. Merck was very aware of these risks and that would be proven in court with internal memos and even unpublished studies showing heart attacks and strokes were six times more likely in Vioxx compared to placebo. Despite that knowledge they would turn into a magician selectively hiding data to make the product appear less dangerous in their VIGOR trial. Even worse, the data they did publish still showed more heart attacks compared to aspirin, but they would double down insisting that aspirin was known to reduce heart attacks. So, the problem was not the Vioxx causing heart attacks, but the pesky aspirin preventing them.
Merck would manage to sell Vioxx for five years to an estimated twenty million patients before voluntarily recalling the drug as the APROVE study would reveal an increased risk of heart attacks. Later the Lancet would estimate that Vioxx caused 88,000 heart attacks of which 32,000 were fatal. Lawsuits would begin piling up and despite several successful trial defenses Merck would establish a settlement fund to the tune of 4.85 billion dollars.
NSAIDS work to reduce inflammation by reducing the activity of cyclooxygenase or Cox enzymes. These enzymes help turn arachidonic acid into prostaglandins. Traditional NSAIDS like aspirin are much more selective for the Cox-1 enzyme by about 170-fold. Decreased prostaglandin production does reduce inflammation but those same prostaglandins have a protective effect on the gut and disrupting them leads to ulcers. The thought was if we could develop more Cox-2 selective drugs we could avoid negative side effects. Vioxx, Bextra, and Celebrex all selective Cox-2 inhibitors would be developed and marketed extensively. Vioxx would be the most advertised prescription product in the year 2000 and Pfizer would promote Bextra for so many off-label indications that it would be part of a 2.3-billion-dollar settlement with the Justice Department for fraudulent marketing. Bextra was removed from the market in 2004 leaving Celebrex as the real winner and the only Cox-2 inhibitor on the market. Celebrex would enjoy essentially a monopoly on the market that was so extensive they would halt advertising the product. Celebrex would stay on the market despite the FDA adding a black box warning to its label.
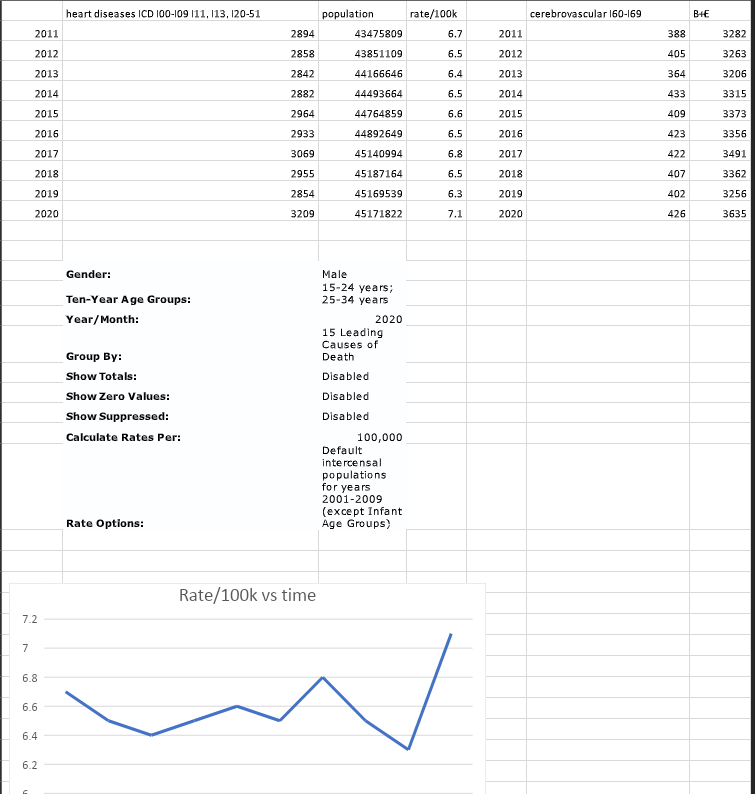
If the vaccines are causing an issue all signs seem to point to the young male age group as where the signal will be found. I have been looking through the CDC database to track the number of reported cardiovascular and cerebrovascular deaths in males between the age of 15-34. Going back to 2011 the amount of these reported deaths is rare and consistent but begins to spike in 2020 the last year we have complete data for. I would bet that this line continues to increase as we get data for 2021 the only question is how much? The good news is that if we are accidentally causing excess heart attacks the Moderna CEO has promised a new breakthrough MRNA treatment to repair damaged heart muscle. God bless his heart for being so far ahead of the curve.
Jacob Hyatt Pharm D.
Father of three, Husband, Pharmacist, Realtor, Landlord, Independent Health, and Medicine Reporter
https://substack.com/discover/pharmacoconuts
Bitcoin GtjoZgxE7WpTkWRE6JiEiXfUpqbWKxH4g
Litecoin ML1N31UVz6sRfo2m2oLaorXgPexUtv3Q3t
www.glenallenliving.com
Additional Reading and References
https://scholarship.law.stjohns.edu/cgi/viewcontent.cgi?article=1054&context=jcred
https://money.cnn.com/2006/09/19/news/companies/celebrex/index.htm
https://pubmed.ncbi.nlm.nih.gov/18030055/
https://www.fdanews.com/articles/75244-black-box-warning-added-to-celebrex-labeling
https://www.npr.org/2007/11/10/5470430/timeline-the-rise-and-fall-of-vioxx